Orrell C. Lancet HIV 2017 4:e536-46
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» INSTI vs PI
» DTG/ABC/3TC vs ATV/rFTC/TDF
Head-to-head comparative trials for first line ART since 2006
» INSTI vs PI
» DTG/ABC/3TC vs ATV/rFTC/TDF
Drugs
DTG, ATV/r, FTC/TDF, ABC/3TC, TDF, ABC, FTC, 3TC
DTG, ATV/r, FTC/TDF, ABC/3TC, TDF, ABC, FTC, 3TC
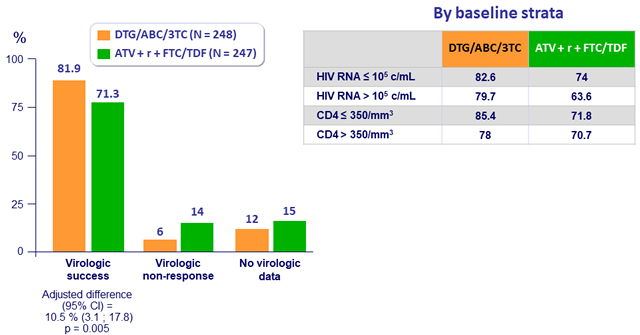
- In treatment-naive women, DTG/ABC/3TC was superior to
ATV + r + TDF/FTC at 48 weeks of treatment- HIV RNA < 50 c/mL (ITT-E, snapshot): 82% vs 71%
- Adjusted difference 10.5%, 95% CI: 3.1% to 17.8%, p = 0.005
- Difference driven by lower rate of virologic non-response and fewer discontinuations due to adverse events in DTG arm
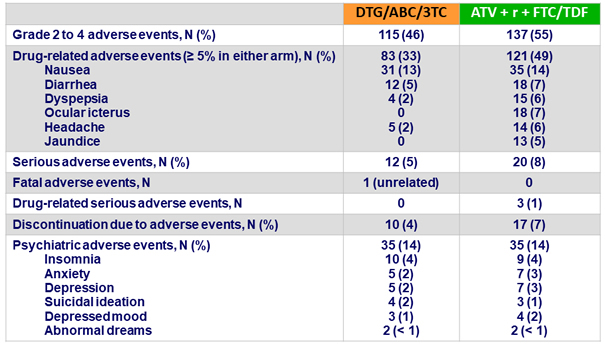
- DTG/ABC/3TC had a favorable safety profile compared to
ATV + r + TDF/FTC
Design
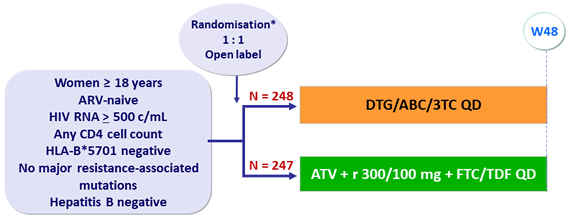
*
Randomisation was stratified by HIV RNA (≤ or > 100 000 c/mL) and CD4 (≤ or > 350/mm3)
Objective
- Non inferiority of DTG/ABC/3TC at W48: % HIV RNA < 50 c/mL by intention to treat, snapshot analysis (lower margin of the 2-sided 95% CI for the difference = - 12%, 90% power)
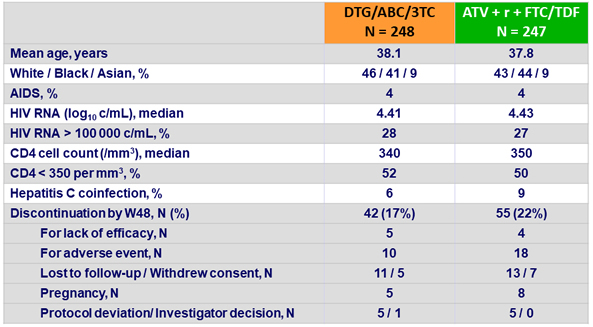
Baseline characteristics and patient disposition
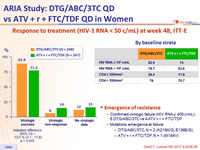
Response to treatment (HIV-1 RNA < 50 c/mL) at week 48, ITT-E
Emergence of resistance
- Confirmed virologic failure (HIV RNA ≥ 400 c/mL): 6 DTG/ABC/3TC vs 4 ATV + r + FTC/TDF
- Mutations emergence at failure
- DTG/ABC/3TC, N = 2 (K219K/Q, E138E/G)
- ATV + r + FTC/TDF, N = 1 (M184V)
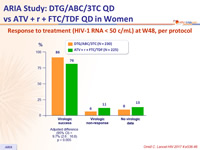
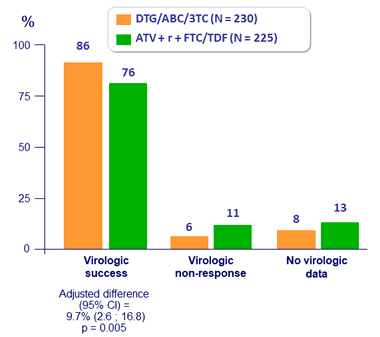
Response to treatment (HIV-1 RNA < 50 c/mL) at W48, per protocol
Adverse events
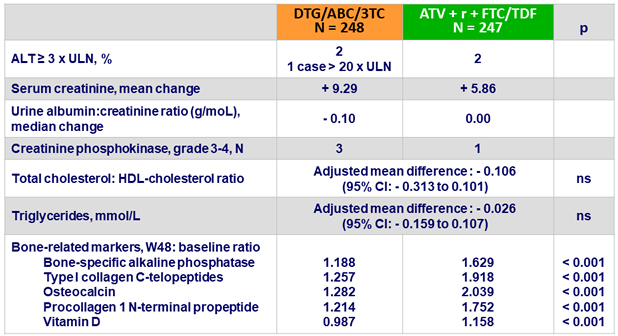
Changes in laboratory parameters at W48