Cahn P. EACS 2009; Abs. PS4/3
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
Drugs
LPV/r, SQV/r, 2 NRTI
LPV/r, SQV/r, 2 NRTI
- LPV/r monotherapy is a potential strategy in virologically suppressed patients on a PI/r-containing regimen
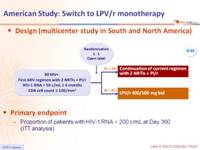
Design (multicenter study in South and North America) :
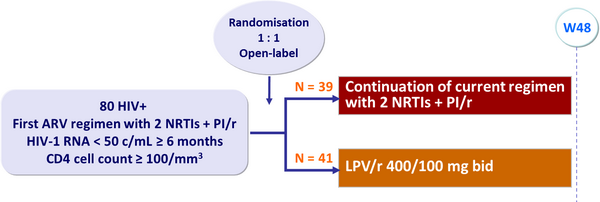
Primary endpoint :
- Proportion of patients with HIV-1 RNA < 200 c/mL at Day 360 (ITT analysis)
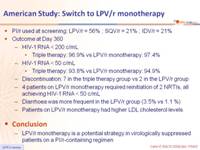
- PI/r used at screening: LPV/r = 56% ; SQV/r = 21% ; IDV/r = 21%
- Outcome at Day 360
- HIV-1 RNA < 200 c/mL
- Triple therapy: 96.9% vs LPV/r monotherapy: 97.4%
- HIV-1 RNA < 50 c/mL
- Triple therapy: 93.8% vs LPV/r monotherapy: 94.9%
- HIV-1 RNA < 200 c/mL
- Discontinuation: 7 in the triple therapy group vs 2 in the LPV/r group
- 4 patients on LPV/r monotherapy required reinitiation of 2 NRTIs, all achieving HIV-1 RNA < 50 c/mL
- Diarrhoea was more frequent in the LPV/r group (3.5% vs 1.1 %)
- Patients on LPV/r monotherapy had higher LDL cholesterol levels