Eron J. AIDS 2018 ;32 :1431-42
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» NRTI combinations
» D/C/F/TAF vs D/C + FTC/TDF
Head-to-head comparative trials for first line ART since 2006
» NRTI combinations
» D/C/F/TAF vs D/C + FTC/TDF
Drugs
DRV/c, FTC/TAF, FTC/TDF, TAF, TDF, FTC
DRV/c, FTC/TAF, FTC/TDF, TAF, TDF, FTC
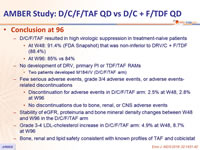
- D/C/F/TAF resulted in high virologic suppression in treatment-naïve patients
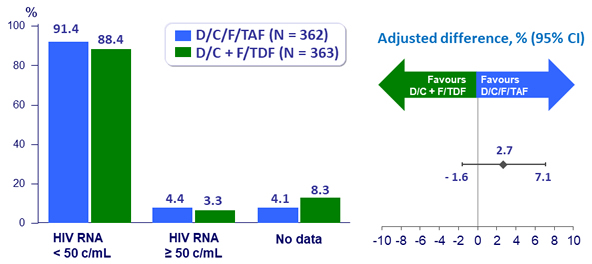
- At W48: 91.4% (FDA Snapshot) that was non-inferior to DRV/C + F/TDF (88.4%)
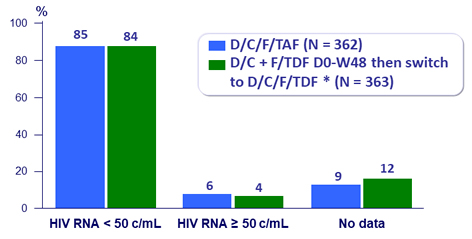
- At W96: 85% vs 84%
- No development of DRV, primary PI or TDF/TAF RAMs
- Two patients developed M184I/V (D/C/F/TAF arm)
- Few serious adverse events, grade 3/4 adverse events, or adverse events-related discontinuations
- Discontinuation for adverse events in D/C/F/TAF arm: 2.5% at W48, 2.8% at W96
- No discontinuations due to bone, renal, or CNS adverse events
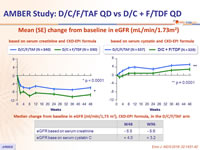
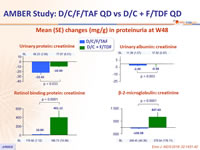
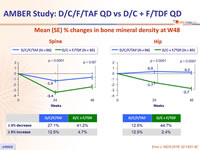
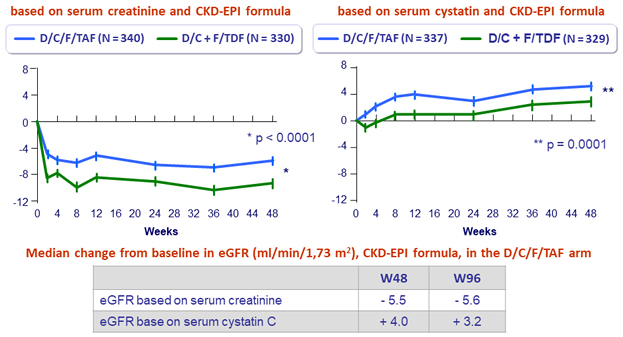
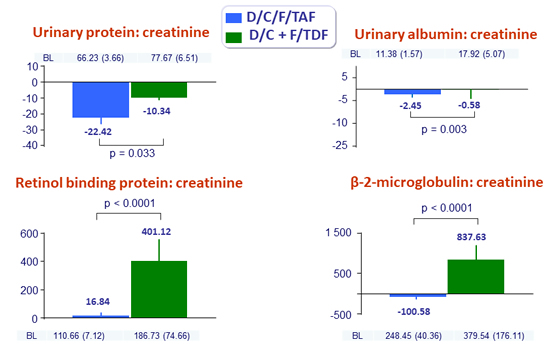
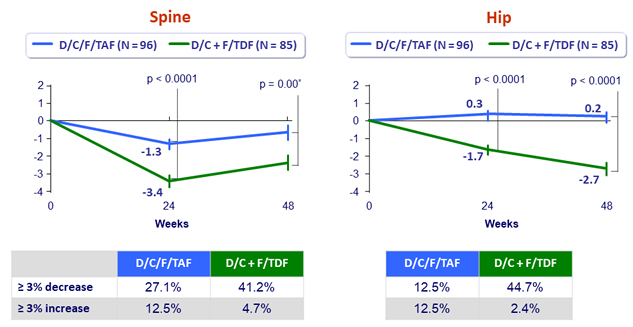
- Stability of eGFR , proteinuria and bone mineral density changes between W48 and W96 in the D/C/F/TAF arm
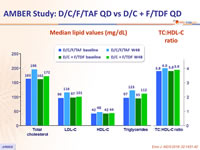
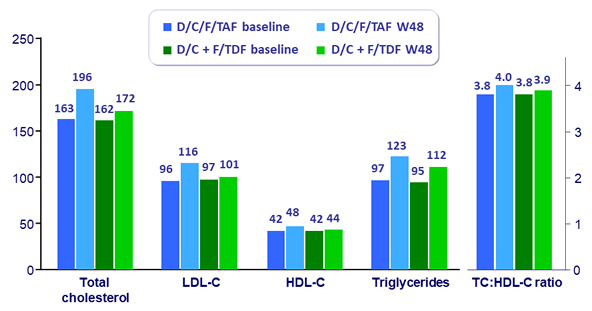
- Grade 3-4 LDL-cholesterol increase in D/C/F/TAF arm: 4.9% at W48, 8.7% at W96
- Bone, renal and lipid safety consistent with known profiles of TAF and cobicistat
Design
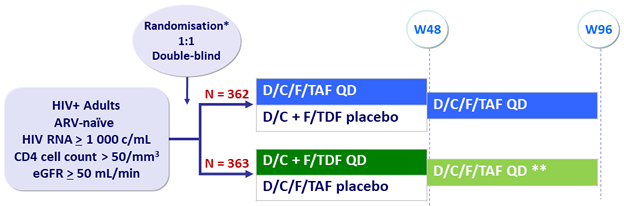
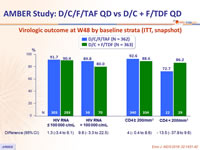
* Randomisation was stratified by HIV RNA (< or ≥ 100 000 c/mL) and CD4 cell count (< or ≥ 200/mm3)
** Patients were switched to open-label D/C/F/TAF after unblinding that occurred at various time after W4
Median duration of exposure (weeks) : D/C/F/TAF = 96.1 ; D/C + F/TDF = 73.1 ; D/C/F/TAF deferred switch = 22.3
Objective
- Non inferiority of E/C/F/TAF at W48: % HIV RNA < 50 c/mL by intention to treat, snapshot analysis (lower margin of the 95% CI for the difference = -10%)
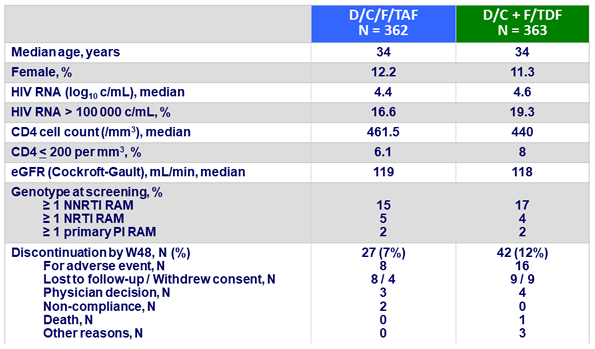
Baseline characteristics and patient disposition
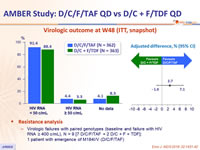
Virologic outcome at W48 (ITT, snapshot)
- Resistance analysis
- Virologic failures with paired genotypes (baseline and failure with HIV RNA ≥ 400 c/mL), N = 9 [7 D/C/F/TAF + 2 D/C + F + TDF]: 1 patient with emergence of M184I/V (D/C/F/TAF)
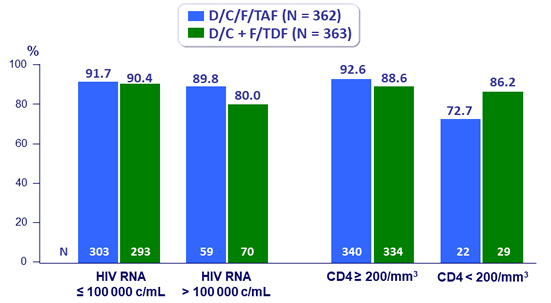
Virologic outcome at W48 by baseline strata (ITT, snapshot)
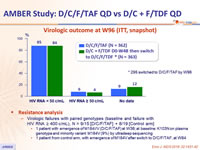
Virologic outcome at W96 (ITT, snapshot)
- Resistance analysis
- Virologic failures with paired genotypes (baseline and failure with
HIV RNA ≥ 400 c/mL), N = 9/15 [D/C/F/TAF] + 8/19 [Control arm]
- 1 patient with emergence of M184I/V (D/C/F/TAF) at W36; at baseline: K103N on plasma genotype and minority variant M184V (9%) by ultradeep sequencing
- 1 patient from control arm, with emergence of M184V after switch to D/C/F/TAF, at W84
- Virologic failures with paired genotypes (baseline and failure with
HIV RNA ≥ 400 c/mL), N = 9/15 [D/C/F/TAF] + 8/19 [Control arm]
Mean (SE) change from baseline in eGFR (mL/min/1.73m²)
Mean (SE) changes (mg/g) in proteinuria at W48
Mean (SE) % changes in bone mineral density at W48
Median lipid values (mg/dL)
Adverse events through W48, %
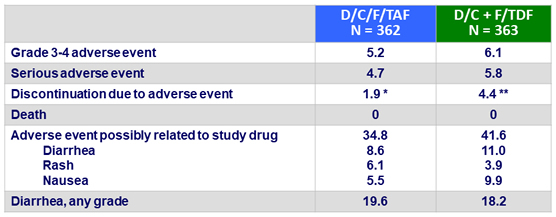
* rash (N = 6) ; diarrhea (N = 1)
** rash/erythema (N = 7), diarrhea (N = 1), toxic skin eruption (N = 2), Stevens Johnson syndrome (N = 1), bone marrow oedema (N = 1), increased beta-2 macroglobulin (N = 1), arthralgia (N = 1), neoplasm (N = 2)
- No grade 3 or 4 laboratory abnormalities in ≥ 5% of patients in either arm
- No discontinuation of D/C/F/TAF for bone, renal or CNS adverse event through W96