Smith KY. AIDS Res Ther. 2008 Mar 28;5:5
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» PI vs PI
» ATV/r + FTC/TDF vs FPV/r + FTC/TDF
Head-to-head comparative trials for first line ART since 2006
» PI vs PI
» ATV/r + FTC/TDF vs FPV/r + FTC/TDF
Drugs
ATV/r, FPV/r, FTC/TDF, TDF, FTC
ATV/r, FPV/r, FTC/TDF, TDF, FTC
- Similar virologic and immunologic outcome at W48 with FPV/r 1400/100 mg QD and ATV/r 300/100 mg QD, in combination with TDF/FTC fdc
- Higher gastrointestinal intolerance with FPV/r
- High incidence of increased bilirubin with ATV/r
- Higher triglycerides increase with FPV/r; total, HDL- and LDL-cholesterol changes similar in both groups
- Limitation: small size of the study
Design :
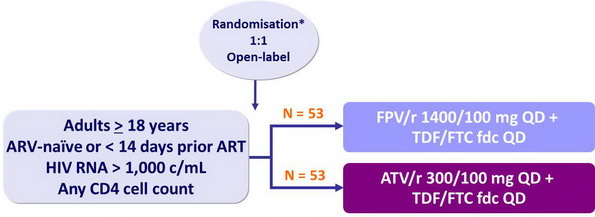
* Randomisation was stratified on HIV RNA < or ≥ 100,000 c/mL
Objective :
- Primary endpoint: HIV RNA < 50 c/mL at W48
- No power calculation due to limited sample size
Note: Â FPV/r and TDF/FTC were administered with or without food; ATV/r with food
Substitution of ABC/3TC fdc for TDF/FTC was allowed
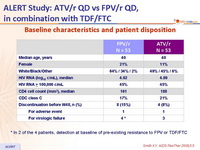
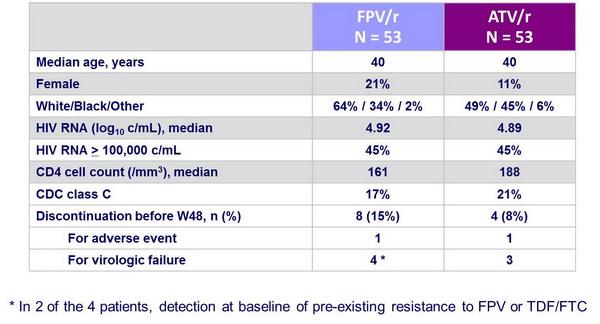
Baseline characteristics and patient disposition :
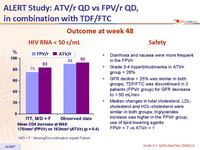
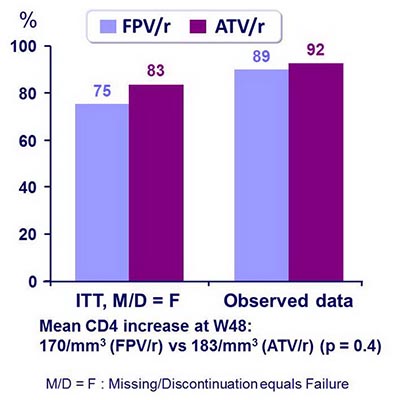
Outcome at week 48 :
HIV RNA < 50 c/mL
Safety
- Diarrhoea and nausea were more frequent in the FPV/r
- Grade 3-4 hyperbilirubinemia in ATV/r group = 28%
- GFR decline > 25% was similar in both groups; TDF/FTC was discontinued in 3 patients (FPV/r group) for GFR decrease to < 50 mL/min
- Median changes in total cholesterol, LDL- cholesterol and HDL-cholesterol were similar in both groups; triglycerides increase was higher in the FPV/r group; use of lipid-lowering agents: FPV/r = 7 vs ATV/r = 1