Lennox JL. Ann Intern Med. 2014 Oct 7;161(7):461-71 ; Ofotokun I. Clin Infect Dis. 2015 Jun 15;60(12):1842-51 ; Brown TT. J Infect Dis. 2015 Oct 15;212(8):1241-9 ; Mc Comsey GA. CROI 2015, Abs. 140 ; Kelesidis T. Clin Infect Dis. 2015 Aug 15;61(4):651-60
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» INSTI vs PI
» ATV/r + FTC/TDF vs DRV/r + FTC/TDF vs RAL + FTC/TDF
Head-to-head comparative trials for first line ART since 2006
» INSTI vs PI
» ATV/r + FTC/TDF vs DRV/r + FTC/TDF vs RAL + FTC/TDF
Drugs
RAL, DRV/r, ATV/r, FTC/TDF, TDF, FTC
RAL, DRV/r, ATV/r, FTC/TDF, TDF, FTC
- ATV/r, RAL, and DRV/r were equivalent for virologic efficacy, when given with TDF/FTC
- ATV/r + TDF/FTC was less-well tolerated than DRV/r + TDF/FTC or RAL + TDF/FTC
- A composite assessment of virologic efficacy and tolerability found that
- RAL + TDF/FTC was superior to both PI-containing regimens
- DRV/r + TDF/FTC was superior to ATV/r + TDF/FTC
- Tolerability result was caused primarily by jaundice for ATV/r and gastrointestinal toxicity for both PI/r
- ATV/r was less tolerated than DRV/r and RAL across all sub-groups
- RAL tolerability benefit over DRV/r was greater in women
- Limitations: open-label design, switch to another arm for tolerability or toxicity allowed
- When tolerability and virologic response are considered together, RAL + TDF/FTC was superior overall to both PI-based therapies and DRV/r was superior to ATV/r. An advantage of PI/r over RAL is the reduced likelihood of drug resistance if virologic failure occurs
Design :
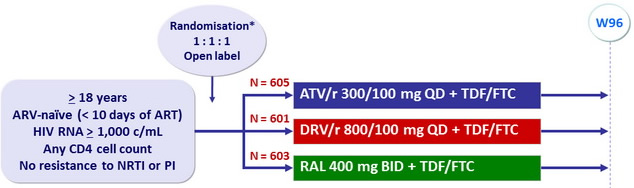
*Randomisation was stratified by HIV RNA (< or ≥ 100,000 c/mL) at screening, participation in cardiovascular sub-study, and 10-year Framingham risk score
Objective :
- Evaluate regimen equivalence regarding virologic efficacy and tolerability over 96 weeks, by intention-to-treat analysis. Equivalence = 2-sided 97.5% CI on the pairwise difference in 96-week cumulative incidence of each individual or composite endpoint falling between - 10% and 10%, 90% power. If equivalence was not shown, superiority was defined as exclusion of 0 from the 97.5% CI
Endpoints :
- Virologic failure: confirmed HIV-1 RNA > 1,000 c/mL at or after W16, or > 200 c/mL at or after W24
- Tolerability failure: time from randomisation to discontinuation of the randomised regimen component for toxicity (substitution of TDF or FTC not considered as tolerability failure)
- Composite endpoint: virologic or tolerability failure, whichever occurred first
- ITT-TLOVR, with HIV-1 RNA threshold of 200 c/mL
- HIV-1 RNA < 50 c/ mL at W96 by ITT, snapshot
- Sensitivity analysis: as-treated (virologic failure including treatment discontinuation as a competing event)
- Key toxicity secondary endpoint: time from initiation of treatment to the first grade 2, 3, or 4 sign or symptom (grade 3 or 4 if after week 48) or any grade 3 or 4 laboratory abnormality while the patient was receiving the randomized treatment (as-treated)
- Prespecified sensitivity analysis excluded hyperbilirubinemia and elevated CK levels
- Further sensitivity analysis included all qualifying adverse events regardless of status on randomized treatment (ITT analysis)
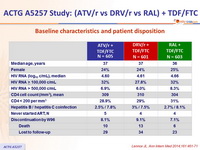
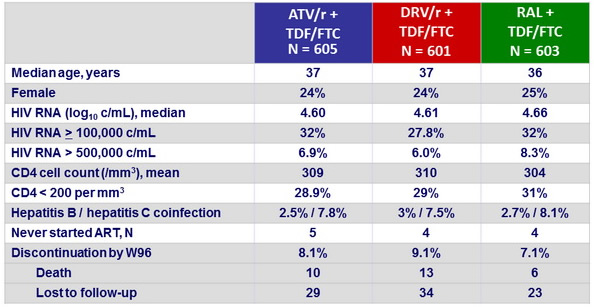
Baseline characteristics and patient disposition :
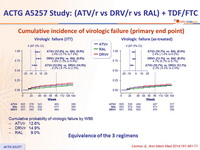
Cumulative incidence of virologic failure (primary end point) :
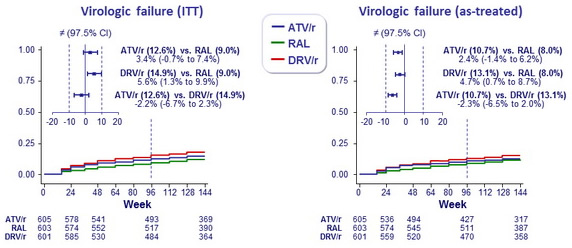
- Cumulative probability of virologic failure by W96
- ATV/r: 12.6%
- DRV/r: 14.9%
- RAL: 9.0%
Equivalence of the 3 regimens
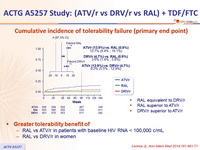
Cumulative incidence of tolerability failure (primary end point) :
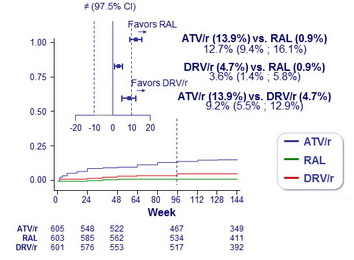
- RAL equivalent to DRV/r
- RAL superior to ATV/r
- DRV/r superior to ATV/r
Greater tolerability benefit of :
- RAL vs ATV/r in patients with baseline HIV RNA < 100,000 c/mL
- RAL vs DRV/r in women
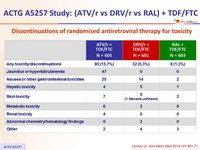
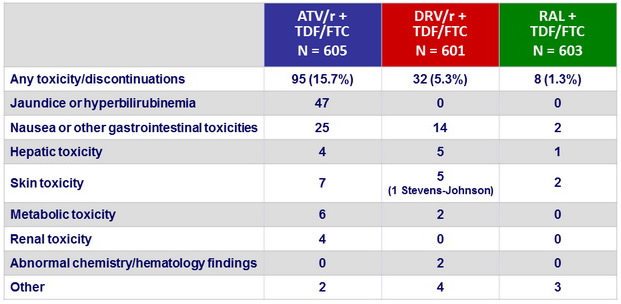
Discontinuations of randomised antiretroviral therapy for toxicity :
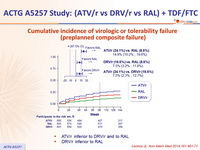
Cumulative incidence of virologic or tolerability failure
(preplanned composite failure) :
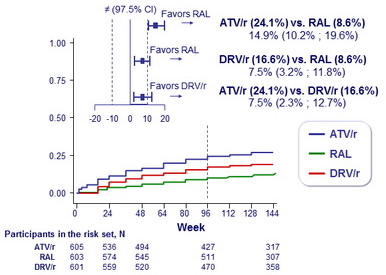
- ATV/r inferior to DRV/r and to RAL
- DRV/r inferior to RAL
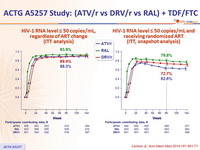
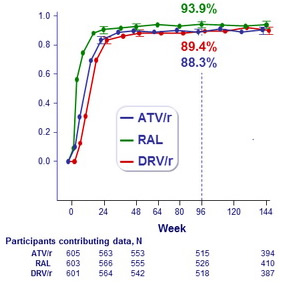
HIV-1 RNA level ≤ 50 copies/mL, regardless of ART change (ITT analysis)
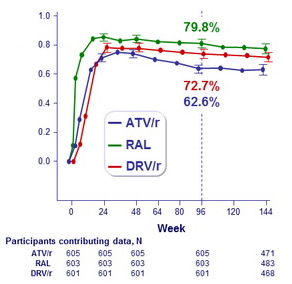
HIV-1 RNA level ≤ 50 copies/mL and
receiving randomized ART
(ITT, snapshot analysis)
Genotypic analysis for resistance at virologic failure :
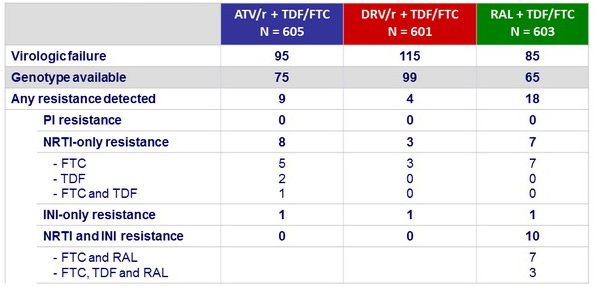
Patients may not have been on their randomised treatment at time of failure
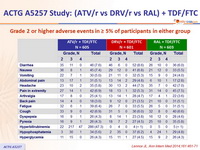
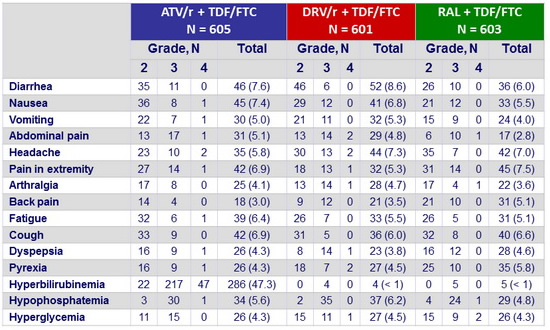
Grade 2 or higher adverse events in ≥ 5% of participants in either group :
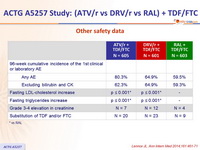
Other safety data :
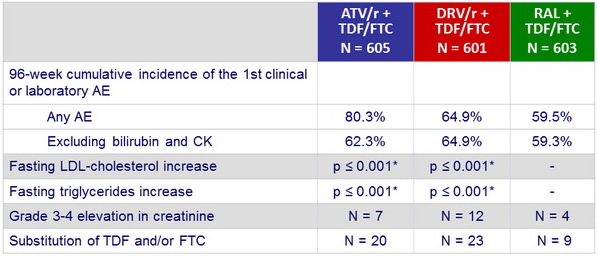
* vs RAL
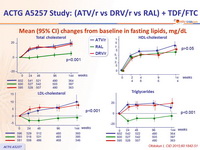
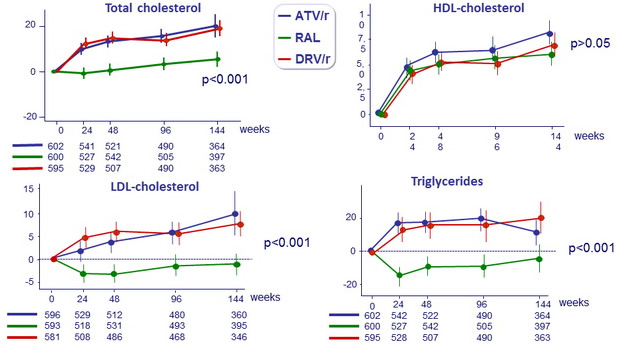
Mean (95% CI) changes from baseline in fasting lipids, mg/ dL
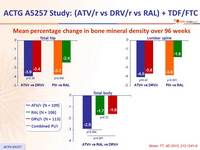
Mean percentage change in bone mineral density over 96 weeks
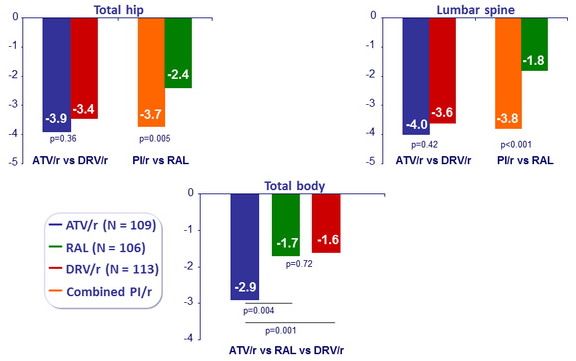
Mean (97.5%) % of body composition change at W96, ITT : limb fat, trunk fat and lean mass (DXA scan), visceral and subcutaneous abdominal fat (CT abdomen)
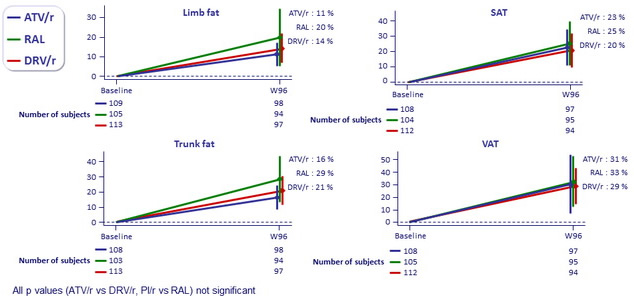
All p values (ATV/r vs DRV/r, PI/r vs RAL) not significant
Larger increases in waist circumference were observed with the RAL arm compared to DRV/r arm
at weeks 48 and 96 (all p ≤ 0.023) but not compared with the ATV/r arm (p ≥ 0.07)
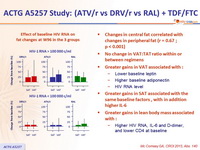
Effect of baseline HIV RNA on fat changes at W96 in the 3 groups
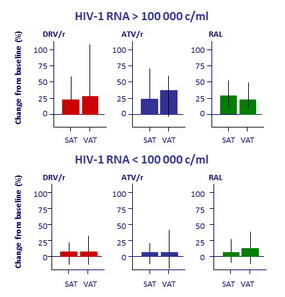
- Changes in central fat correlated with changes in peripheral fat (r = 0.67 ; p < 0.001)
- No change in VAT:TAT ratio within or between regimens
- Greater gains in VAT associated with :
- Lower baseline leptin
- Higher baseline adiponectin
- HIV RNA level
- Greater gains in SAT associated with the same baseline factors, with in addition higher IL-6
- Greater gains in lean body mass associated with :
- Higher HIV RNA, IL-6 and D- dimer, and lower CD4 at baseline
Changes in Inflammation and Immune activation
- Substudy A5260S (328 patients) : 234 included (HIV RNA < 50 c/mL
at W24) : 68 on ATV/r, 84 on DRV/r and 82 on RAL
- Plasma biomarkers of inflammation and coagulation : hsCRP, IL-6, GlycA, D- dimer, sCD14, sCD163, and sIL-2r
- Blood cellular markers : %CD38+DR+ of T-cell subsets and %CD14+CD16+ and %CD14(dim)CD16+ monocyte subsets
- Changes in biomarkers varied by regimen during the 96 weeks of follow-up :
- hsCRP declined with ATV/r and RAL
- IL-6 declined only with RAL
- GLycA decreased in all groups
- D-dimer declined with ATV/r and DRV/r and was unchanged with RAL
- Markers of T-cell activation and sCD163 (but not sCD14 and CD14-+CD16+) declined in all groups
- Conclusion : no consistent evidence that the reduction of inflammation and immune activation with ART initiation was different between RAL and PI-based regimens