Riddler SA. N Engl J Med. 2008 May 15;358(20):2095-106
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» NNRTI vs PI/r
» EFV + 2 NRTI vs LPV/r + 2NRTI vs EFV + LPV/r
Head-to-head comparative trials for first line ART since 2006
» NNRTI vs PI/r
» EFV + 2 NRTI vs LPV/r + 2NRTI vs EFV + LPV/r
Drugs
LPV/r, EFV 600, 2 NRTI
LPV/r, EFV 600, 2 NRTI
- 96-week randomized trial comparing 3 regimens for initial therapy for HIV infection
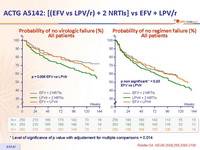
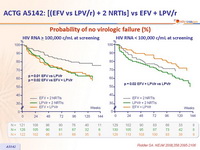
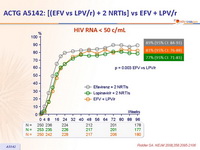
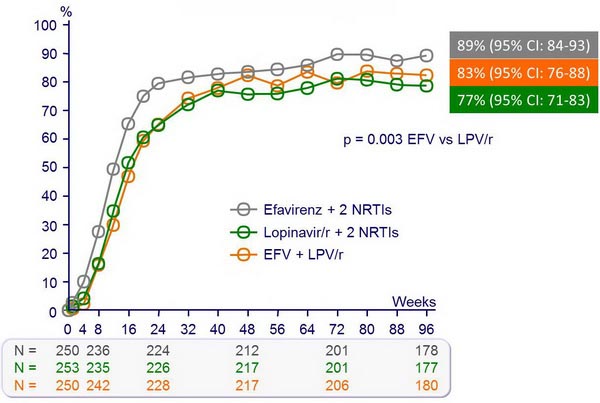
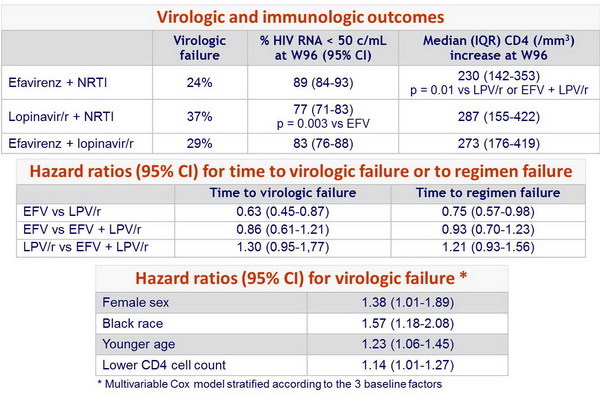
- Less virologic failure with EFV + 2 NRTIs than with LPV/r + 2 NRTIs
- NRTI-sparing regimen of EFV + LPV/r: virologic efficacy similar to EFV + 2 NRTIs but more frequent NNRTI resistance and lipid abnormalities
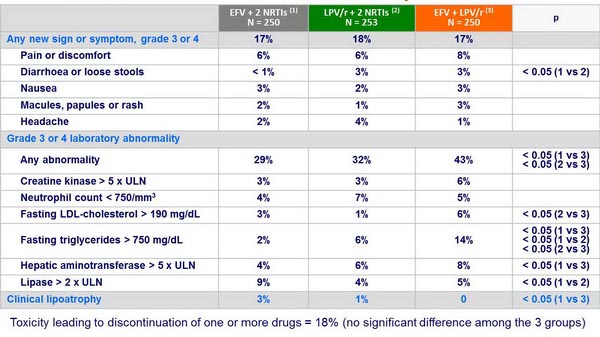
- Non-significant trend toward a shorter time to regimen failure with LPV/r + 2 NRTIs as compared with EFV + 2 NRTIs
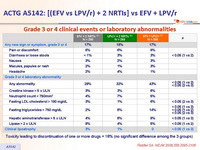
- No significant difference among the 3 groups in the time to treatment-limiting toxicity
- Lower increases in CD4 count with EFV + 2 NRTIs compared to the 2 LPV/r groups
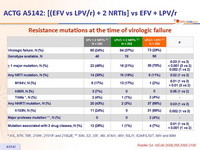
- Resistance emergence: NRTI resistance frequency not significantly different between EFV + 2 NRTIs and LPV/r + 2 NRTIs; mutations to 2 drug classes significantly more frequent with EFV + 2 NRTIs; EFV + NRTI failure associated with high frequency of NNRTI resistance; failure of LPV/r + 2 NRTIs not associated with LPV resistance
- This study shows moderate efficacy superiority of EFV + 2 NRTIs as compared with LPV/r + 2 NRTIs for initial therapy of HIV-1 infection
- Results highlight the complexity of choosing initial therapy with the need to take into considerations many factors, including virologic and immunologic response, tolerability, short-term and long-term toxicity, and the resistance consequences associated with virologic failure
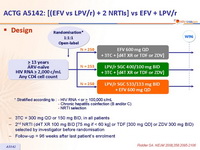
Design :
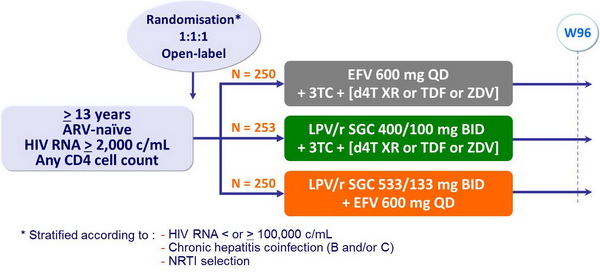
- 3TC = 300 mg QD or 150 mg BID, in all patients
- 2nd NRTI (d4T XR 100 mg BID [75 mg if < 60 kg] or TDF [300 mg QD] or ZDV 300 mg BID) selected by investigator before randomisation
- Follow-up = 96 weeks after last patient's enrolment
Objectives :
- Time to virologic failure: lack of suppression of HIV RNA by 1 log10 c/mL or rebound before W32, or lack of suppression of HIV RNA < 200 c/mL, or rebound after W32. Confirmation of suspected virologic failure was required within 4 weeks. If confirmation sample was missing, case was included as failure
- Time to regimen failure: first of either virologic failure or toxicity-related discontinuation of any component of the initial randomized treatment regimen
Analyses :
- ITT analyses stratified according to the 3 randomisation factors, including all patients
- who received at least one dose of study drug
- If discontinuation for intolerance, follow-up continued for the occurrence of virologic failure
- If no virologic nor regimen failure, data was censored at last study visit
- Missing data due to missed evaluations, loss to follow-up, or censoring were ignored
- Power of 85% to detect a 56% reduction in the risk of virologic failure
- Power of 90% to detect a 52% reduction in the risk of regimen failure
- Primary endpoints assessed with Kaplan-Meier (statistical significance of hazard ratios between study groups: p < 0.014)
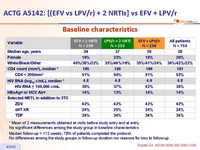
Baseline characteristics :
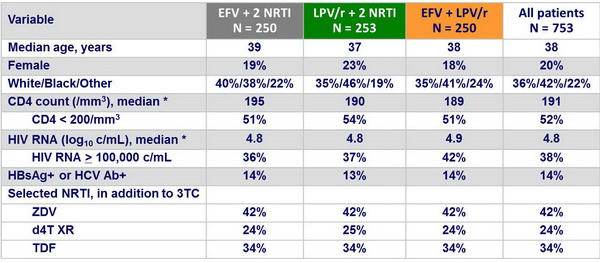
* Mean of 2 measurements obtained at visits before study entry and at entry
No significant differences among the study group in baseline characteristics
Median follow-up = 112 weeks; 78% of patients completed the protocol.
No differences among the study groups in follow-up duration nor reasons for loss to follow-up
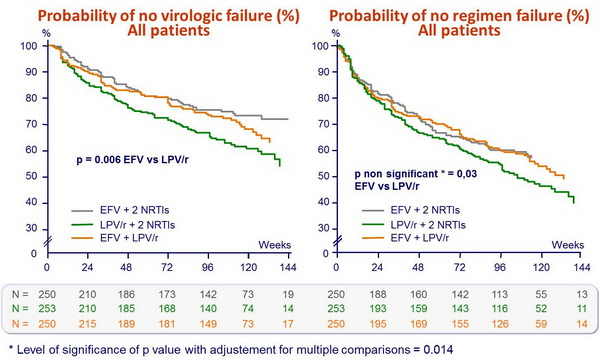
Probability of no virologic failure (%) :
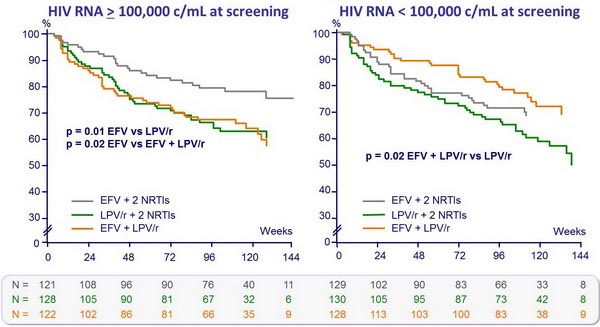
HIV RNA < 50 c/mL :
Grade 3 or 4 clinical events or laboratory abnormalities :