Mills A. J Acquir Immune Defic Syndr. 2013 Feb 1;62(2):164-70
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» 2 drugs vs 3 drugs
» NRTI-Sparing
» ATV/r + MVC vs LPV/r + FTC/TDF
Head-to-head comparative trials for first line ART since 2006
» 2 drugs vs 3 drugs
» NRTI-Sparing
» ATV/r + MVC vs LPV/r + FTC/TDF
Drugs
MVC, ATV/r, LPV/r, FTC/TDF, TDF, FTC
MVC, ATV/r, LPV/r, FTC/TDF, TDF, FTC
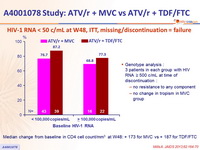
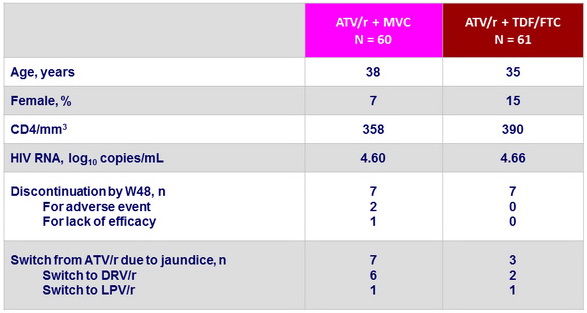
- This open-label study in treatment-naive patients with CCR5-tropic virus showed that a high proportion of patients in the MVC and TDF/FTC treatment groups achieved and maintained viral suppression through 48 weeks of treatment
- Low potential for resistance or loss of susceptibility to study drugs at treatment failure
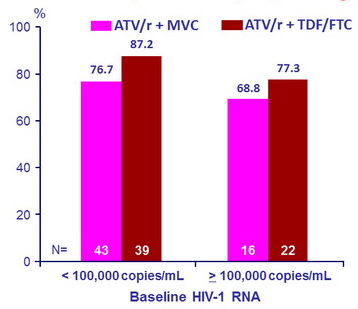
- When stratified by plasma HIV-1 RNA concentration at baseline, the number of patients who achieved plasma HIV-1 RNA < 50 c/mL at W48 was higher in the TDF/FTC + ATV/r treatment arm compared with the MVC + ATV/r group
- CD4 cell counts increased from baseline in both treatment groups
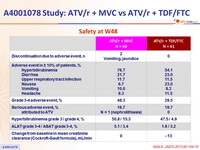
- The frequency of treatment-limiting hyperbilirubinemia was greater than expected
- Limitations
- Unpowered to establish non-inferiority
Design
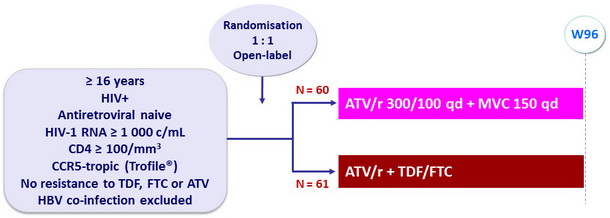
Objective
- Primary endpoint: % with HIV RNA < 50 c/mL at W48 (ITT, missing, discontinued = failure), not powered to show a difference
- Protocol-defined treatment failure: < 1.0 log10 c/mL decrease from baseline in plasma HIV RNA at W4 or thereafter; failure to achieve plasma HIV RNA < 400 c/mL at W24; or rebound in plasma HIV RNA > 1 000 c/mL on 2 consecutive measurements ≤ 14 days apart in patients having achieved levels < 400 c/mL on 2 consecutive visits
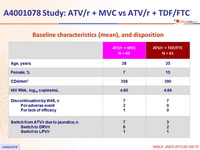
Baseline characteristics (mean), and disposition
HIV-1 RNA < 50 c/mL at W48, ITT, missing/discontinuation = failure
Genotype analysis :
3 patients in each group with HIV RNA ≥ 500 c/mL at time of discontinuation :
- no resistance to any component
- no change in tropism in MVC group
Median change from baseline in CD4 cell count/mm 3 at W48: + 173 for MVC vs + 187 for TDF/FTC
Safety at W48