Mills A. JAIDS 2015; 69: 439-45
Type of ARV Trial
Phase 2 of new ARVs
» TAF (TFV prodrug)
» D/C/F/TAF vs D/C + FTC/TDF
Phase 2 of new ARVs
» TAF (TFV prodrug)
» D/C/F/TAF vs D/C + FTC/TDF
Drugs
D/C/F/TAF, DRV/c, FTC/TAF, FTC/TDF, TAF, TDF, FTC
D/C/F/TAF, DRV/c, FTC/TAF, FTC/TDF, TAF, TDF, FTC
- In this phase 2, randomised clinical trial, HIV-positive treatment-naive adults received STRs of E/c/F/TAF or E/c/F/TDF. Both STRs demonstrated high and comparable rates of virologic suppression through 48 weeks of therapy
- Both regimens were well tolerated, with few discontinuations due to adverse events. Nausea occurred more frequently with E/c/F/TAF
- Plasma concentrations of TFV were substantially (91%) lower with E/c/F/TAF than with E/c/ F/TDF, and the TAF regimen delivered 5.3 times the intracellular, physiologically active metabolite, TFV-DP, to PBMCs, which could translate into less end-organ toxicity and/or improved virologic control
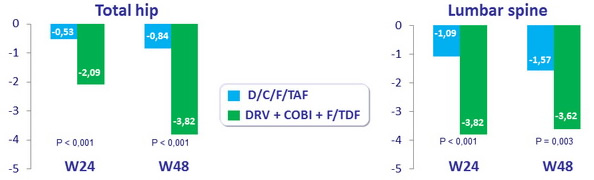
- Significant smaller decreases in bone mineral density through 48 with E/c/F/TAF than with E/c/F/TDF
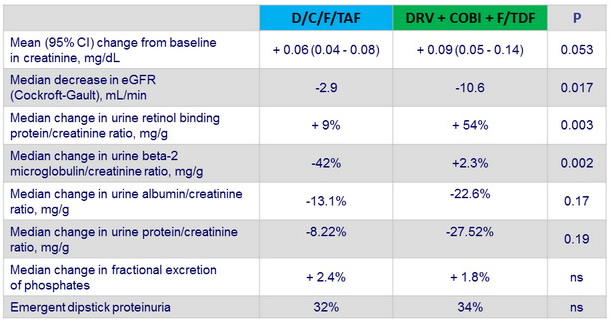
- Urinary RBP/creatinine and b-2 microglobulin/creatinine ratios were significantly lower in the E/c/F/TAF arm, which suggests that TAF has a lesser effect than TDF on the proximal renal tubular cell
Design
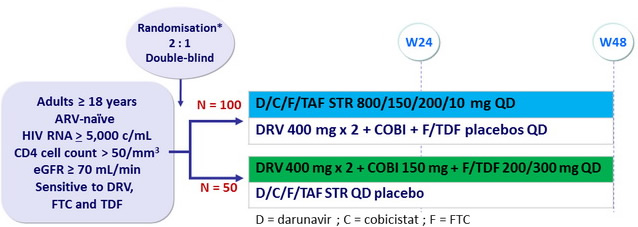
* Randomisation was stratified by HIV RNA ( < or > 100,000 c/mL ) and race (black or nonblack)
Objective
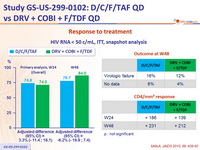
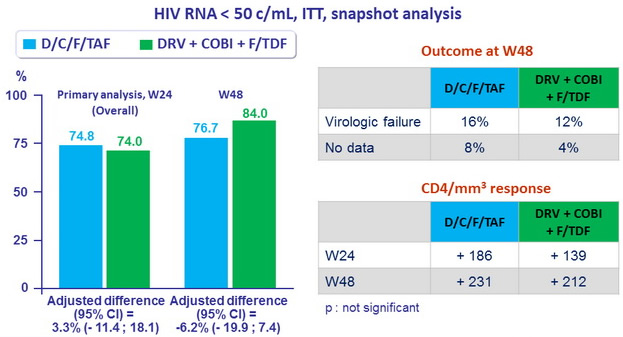
- Primary endpoint : non inferiority of D/C/F/TAF at W24: % HIV RNA < 50 c/ mL by intention to treat, snapshot analysis (lower margin of the 2 sided 95% CI for the difference = -12%)
- Secondary endpoints : HIV RNA < 50 c/ mL at W48, safety, tolerability
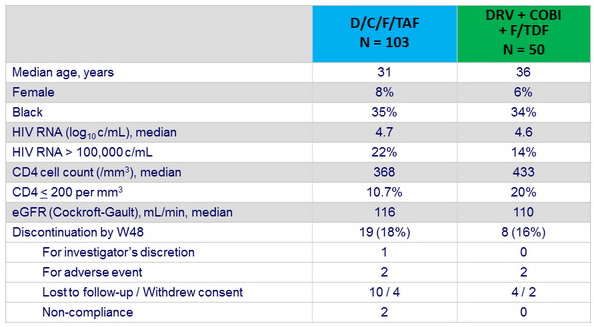
Baseline characteristics and patient disposition
Response to treatment
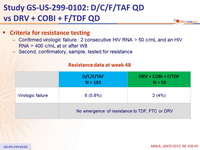
Criteria for resistance testing
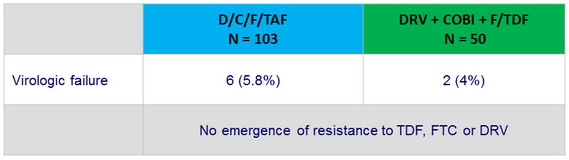
- Confirmed virologic failure : 2 consecutive HIV RNA > 50 c/ mL and an HIV RNA > 400 c/ mL at or after W8
- Second, confirmatory, sample, tested for resistance
Resistance data at week 48
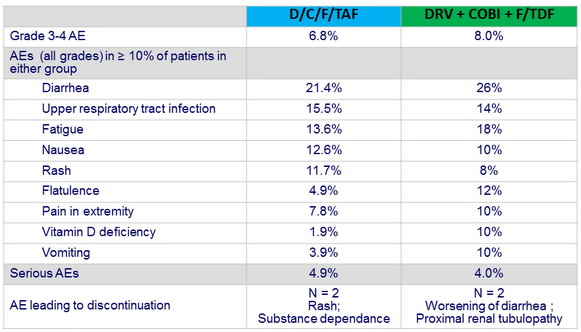
Adverse events by W48
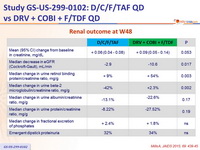
Renal outcome at W48
Mean percentage change from baseline in bone mineral density (DEXA)
Bone sub-study outcome at W48
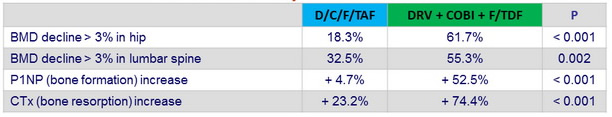
P1NP : pro-collagen Type 1 N-terminal propeptide ; CTx : C-terminal telopeptide
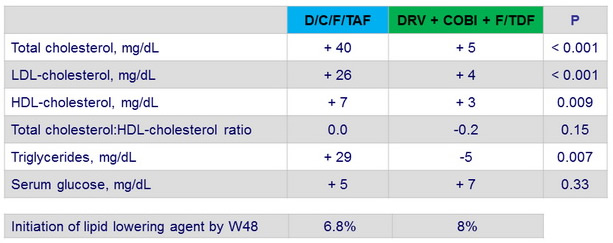
Median change in fasting metabolic parameters at Week 48